New anti-cancer therapy with high efficacy
Researchers at Stanford University School of Medicine have developed a new two-component immunotherapy against cancer. During tests on mice, the method yielded excellent results. The work of American scientists may prove to be a breakthrough in cancer treatment.
Com The cells of our immune system are able to destroy com cancer, but often cancers can suppress their activity, so they need a stimulus to do so. A study on mice conducted at Stanford University describes a new way to b on activation of the immune system to carry out attacks In the fight against drug-resistant bacteria, our immune system can recreate the entire body and replace virtually every missing type of com.
The method involves injecting a mixture that stimulates the immune system directly into the tumor. In this way, the body destroys not only cancer r, in which ry was directly injected with a cocktail that activates the action of the com rek of the immune system, but also other tumors.
According to the authors of the study published in the „Science Translational Medicine”, The new therapy is fast and relatively cheap. Importantly, it does not cause any effects of in side. It also seems to work on many torts in cancer .
One of the authors of the of the new method is Professor Ronald Levy of Stanford University in Palo Alto, California. Together with Idit Sagiv-Barfi and co University researchers have developed a two-component cocktail, which they ry effectively activate com immune cells.
Levy is a pioneer in the field of immunotherapy, which rej scientists pr They are using the immune system to fight cancer. It was his work that led to the development of rituximab, one of the first so-called “anti-inflammatory” drugs. Monoclonal antibodies, which re has been approved for use in anti-cancer therapies.
Some re previously developed immunotherapy methods involve stimulating the immune system throughout the body. Others are targeting the so-called. immune response checkpoints, kt re reduce the anticancer activity of the com immune re. Still others, require removal of the com re of the patient’s immune system from the body and modifying them to attack cells when reintroduced into the body tumor cells.
Many of these therapies have significant successes to their credit, but they all have drawbacks. From difficult to control the effects of in side after costly and lengthy treatment or therapy preparation times.
– All these advances in immunotherapy are changing the practice of medicine. Our approach uses a single application of very small amounts of dw ch measures for stimulation of the com immune responses only within the tumor itself. In mice, we observed amazing, og ln systemic effects, including elimination of tumor ithin the whole organism – admitted Levy.
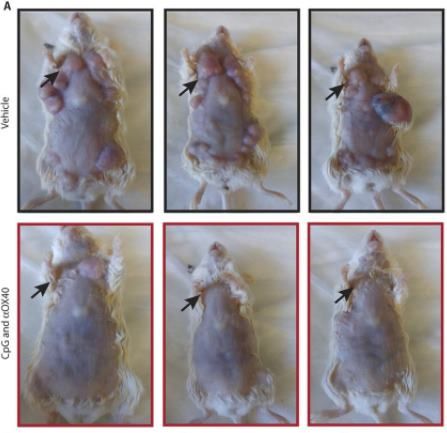
fot. Sagiv-Barfi et al./Science Translational Medicine
According to the new method, „kick” into action the immune system is given two substances injected directly into the tumor. T lymphocytes – com rks of the immune system, although they recognize the lesion, but their action is suppressed by the tumor.
Introduction directly into the tumor of small amounts of CpG oligonucleotide, which ry enhances the expression of the 0X40 receptooa on the surface of the com T cells and an antibody that binds 0X40 and activates the T cells to act against the cellular rk tumor, yields remarkable results. At least in mice.
As a result of such therapy, T lymphocytes are forced to react against the tumor. Some of the activated com immune sharks leave the cancerous lesion where they were injected and can effectively destroy metastases throughout the body.
Laboratory tests showed outright unheard-of efficacy. Of the 90 mice suffering from lymphoma, on kt rych the method was tested, 87 completely recovered. In addition, opr cz lymphoma each of the rodents had metastasized, which re also successfully cured. In several cases, there was a relapse in the disease, but another injection solved the problem.
Importantly, one of the measures used in therapy has already been approved for use in humans during other studies. The other has been tested repeatedly during tests in clinical, suggesting that its approval is a matter of time. All this shortens the time to apply the new method in humans.


